We don’t just build products, we build companies.
Your Vision, Our Mission.
To foster our clients’ vision, we design, develop and deliver market-ready devices that ensures revenue and growth.
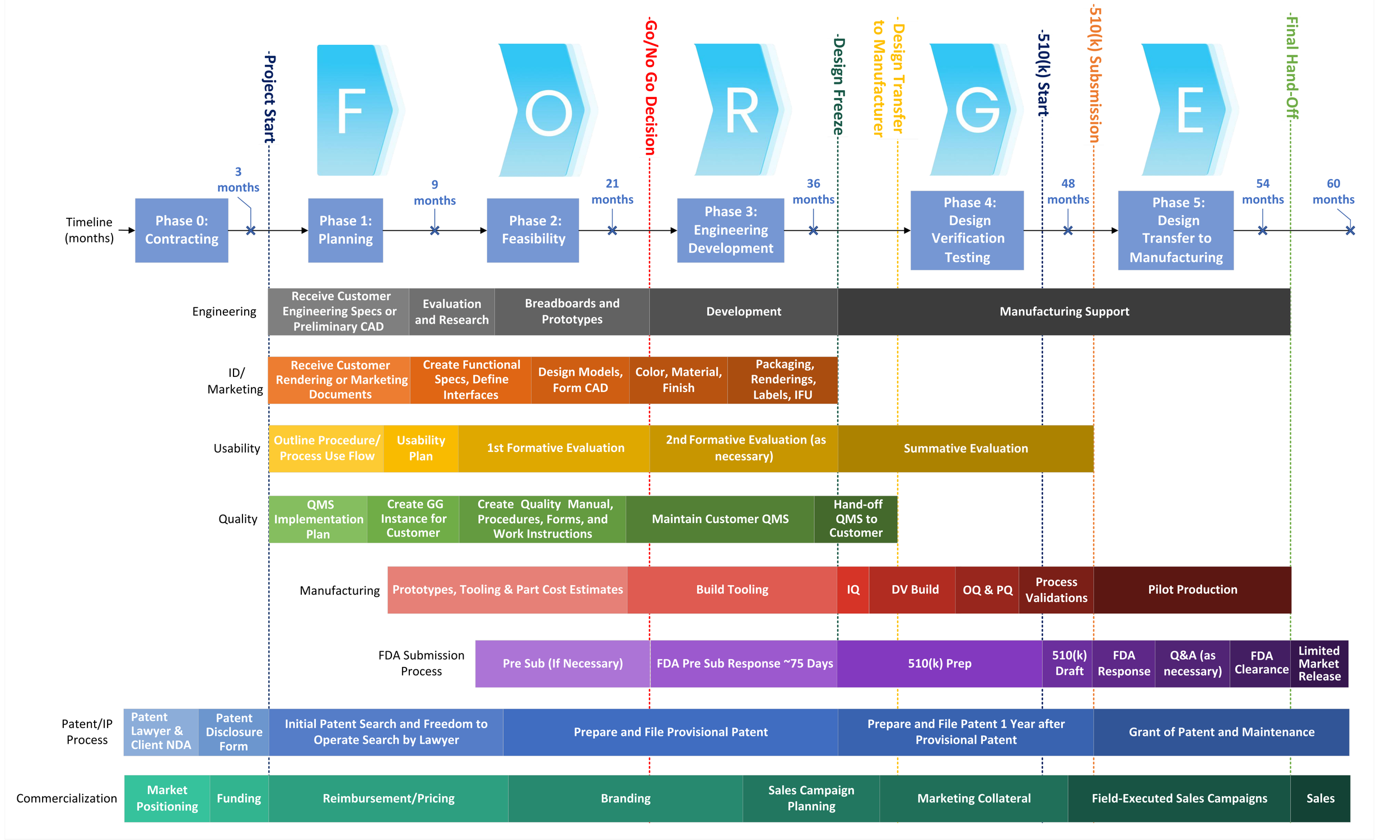
HaA’s Process Flowchart
F O S T E R
Align your concept with market needs and regulatory pathways.
We help you define user needs, identify risks early, and create a clear plan to move forward.
OUTCOME:
We establish a clear roadmap, define the project’s goals, allocate resources, and create a timeline for development. Comprehensive risk assessments and regulatory strategies are also formulated to ensure compliance with relevant standards and regulations.
O P T I M I Z E
Design solutions with compliance, scalability, and manufacturing in mind.
From UI to industrial design, we develop devices that look good, feel intuitive, and are built to scale.
OUTCOME:
This stage involves conducting market research, evaluating the technical feasibility, immersion into intellectual property and assessing potential risks and challenges. The goal is to ascertain whether the concept is worth pursuing, considering factors such as market demand, technological capabilities, & cost-effectiveness.
R E F I N E
Improve processes, tooling, and automation to reduce costs and maximize device reliability.
We help optimize designs and workflows before moving to manufacturing.
OUTCOME:
This stage focuses on the actual design and development of the device, involving the creation of detailed engineering specifications, prototypes, and initial testing. Collaboration between engineers, designers and other experts is essential to refine the device’s design and ensure it meets performance and safety requirements.
G U I D E
Navigate regulatory hurdles and secure your IP and lock in your competitive edge.
From Human Factor studies to 510(k) submissions, we make sure nothing stands between you and your approval.
OUTCOME:
This stage ensures the product is ready for further evaluation and regulatory approval.
E X E C U T E
Deliver a safe, effective, market-ready device with full FDA clearance to launch with confidence.
We bring your innovation across the finish line—ready for real-world use, scaling, and growth.
OUTCOME:
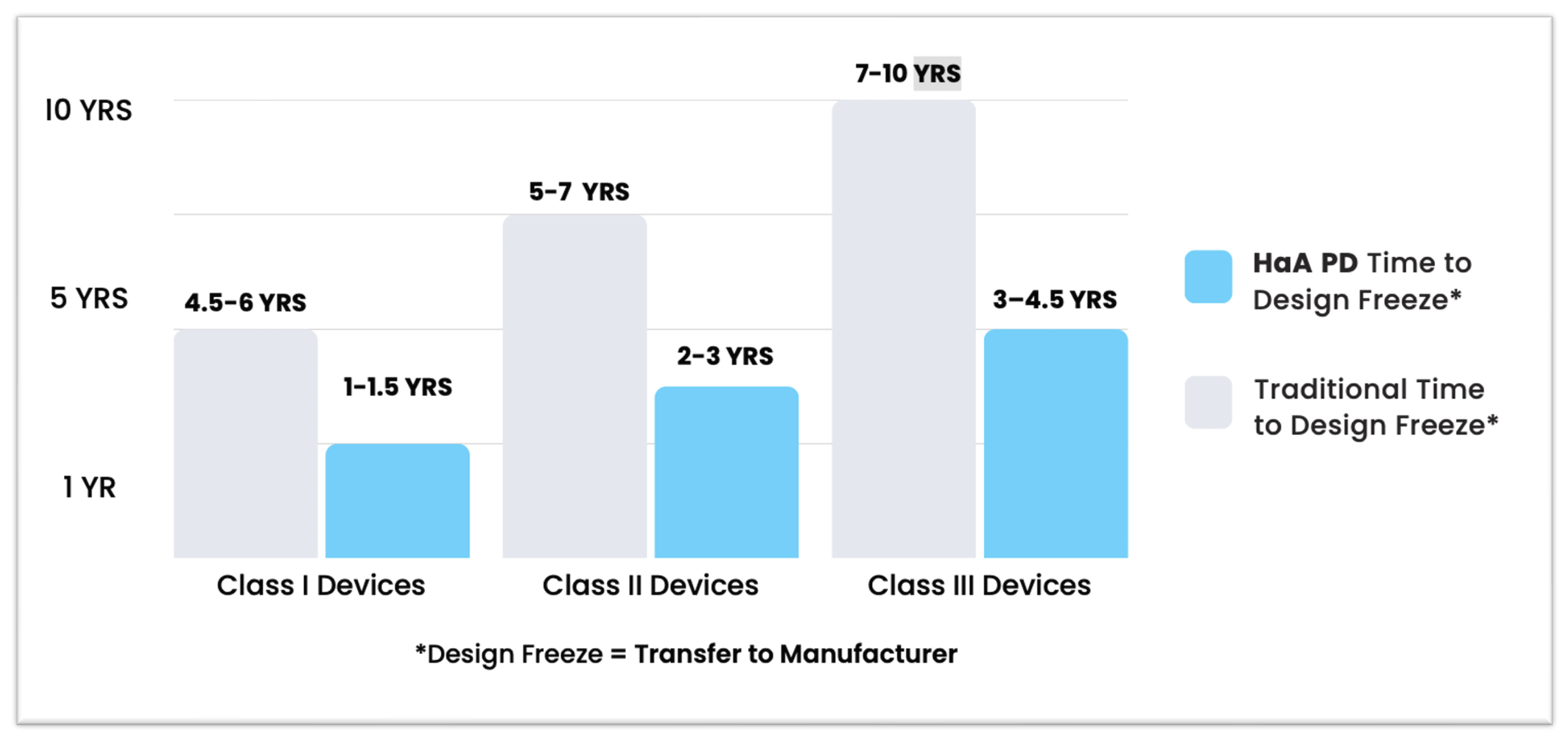
The final stage of the medical device development process involves transferring the validated design to manufacturing for mass production with complete documentation packages and costed Bill of Materials (BOM).