RadiaDyne’s Oncology Tools
Advancing Prostate and Ovarian Cancer Treatment
Problem
Radiation oncologists face challenges in protecting critical organs and monitoring doses in real-time. RadiaDyne, in collaboration with HaA PD, sought to address this with an innovative solution, improving patient outcomes while reducing procedure time and cost.
Radiadyne, after years of research with MD Anderson, needed a cutting-edge fiber optic dosimeter that measured radiation doses in real time, without correction factors. They also wanted a reusable sensor to reduce treatment costs across multiple cancer therapies.
Starting from a napkin sketch, we helped develop RadiaDyne’s IP, creating advanced radiation oncology tools that offer virtual real-time in-vivo dose monitoring, improving stability and safety during therapy.
Solution
HaA Product Development partnered with the client to design and engineer three complementary devices, each addressing a distinct clinical requirement:
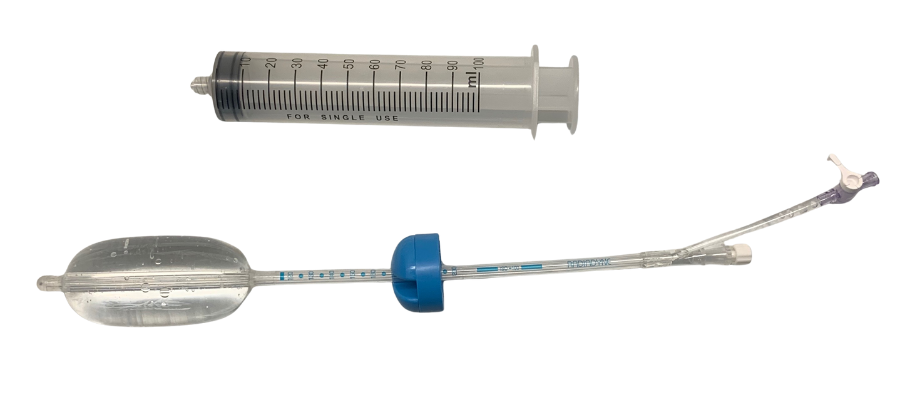
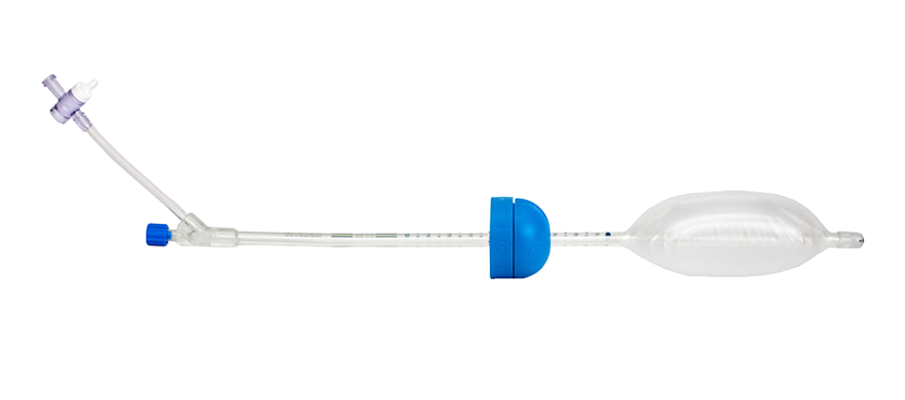
1. Prostate Stabilization Device (ImmobiLoc® / Prostate Immobilizer Rectal Balloon) - A rectal balloon system developed to stabilize the prostate during radiation therapy. The design provided consistent, repeatable positioning of the prostate using an inflatable rectal balloon; radiopaque markers and dielectric features for clear visualization under CT scan; and materials and geometry optimized for patient comfort, clinician usability, and imaging clarity
2. Vaginal/Ovarian Stabilization Device (ALATUS Vaginal Balloon Packing System) - This device is for ovarian and gynecologic radiation treatments. HaA engineered an inflatable vaginal/ovary stabilization balloon capable of reproducible positioning of the vaginal canal and surrounding organs; creating an anatomically appropriate buffer to protect adjacent tissue; and integrating radiopaque indicators for accurate CT-based treatment planning
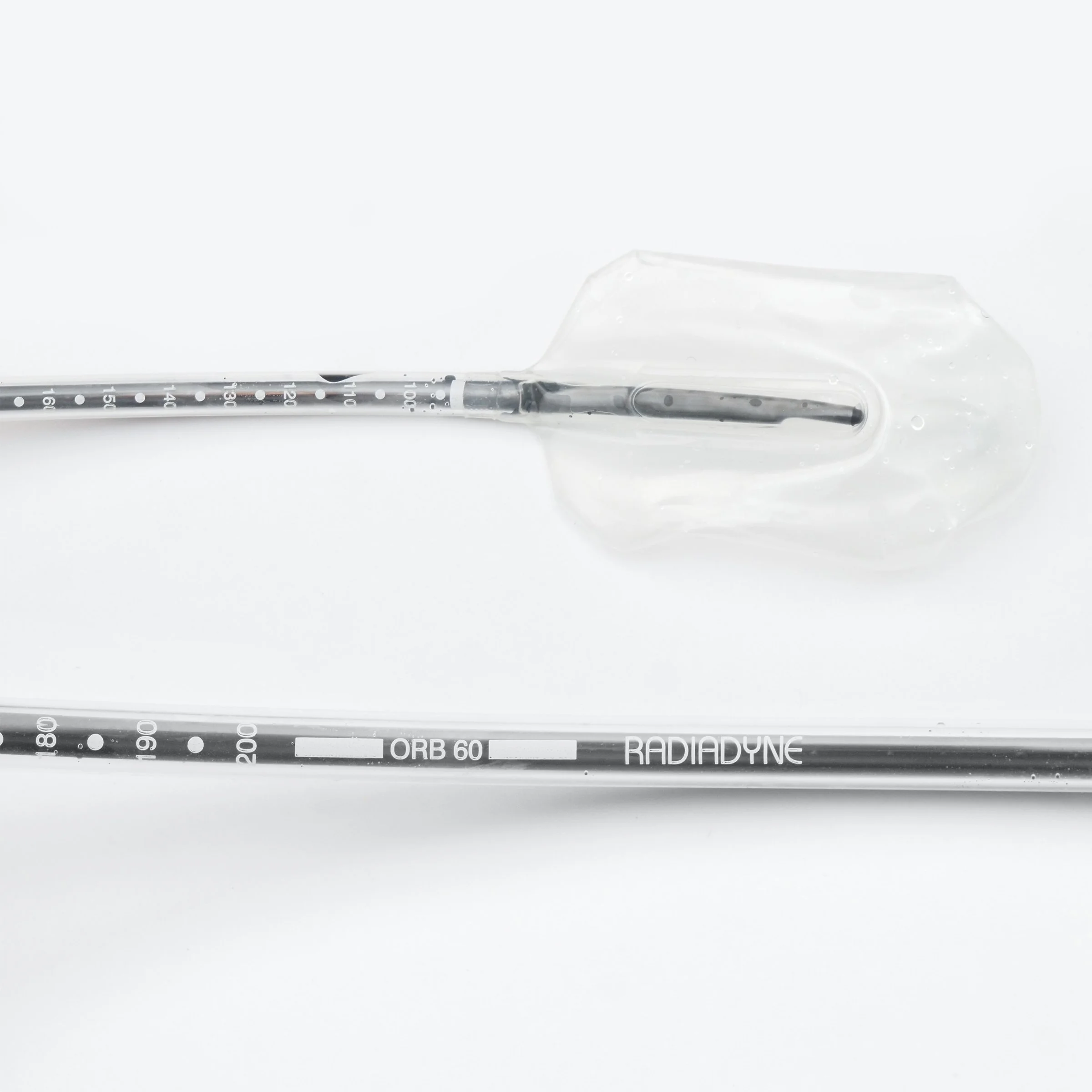
3. Integrated Fiber-Optic Radiation Sensor - A miniature radiation sensor using scintillating fibers coupled to an optical fiber was designed for use with both stabilization devices. The sensor could also be externally taped to the skin for head-and-neck or thyroid applications where no internal balloon was needed. Key technical elements included scintillating fiber that produces light proportional to delivered radiation dose; an optical fiber connection to the Radiadyne Ortrac optical system; dual-reading capability allowing calculation of both radiation dose and dose rate (velocity); and a compact, flexible assembly suitable for embedding in the balloon catheters
The RadiaDyne Radiation Dose Monitoring optical system development was managed under a separate project, ensuring full performance alignment between the device hardware, sensor output, and clinical workflow.
Outcome
The program resulted in a fully integrated set of stabilization and sensing solutions that significantly improved clinical accuracy and treatment confidence. Together, the three-product suite advanced the precision, monitoring, and safety of radiation therapy across multiple cancer types. This innovation not only improved patient care, but also contributed to RadiaDyne’s acquisition by AngioDynamics in 2018.
Services Provided to Develop RadiaDyne’s Oncology Tools
Research
HaA conducted an alignment exercise that uncovered many questions related to the product, procedure, and the sources of information within the company. This helped to focus the team on what answers were needed and where those answers would be obtained.
Analyzed failure modes in current procedures and ensured that we captured critical needs.
HaA helped the company design these products starting with napkin sketches all the way through final prototypes and manufacturing.
Usability
Reviewed procedural videos and documentation to evaluate product usage.
Identified unmet needs in the market to ensure the product met the needs. required for success.
Multiple usability feedback sessions were conducted with a range of physicians.
Workflow Optimization
Mapped the full surgical procedure to pinpoint failure points.
Streamlined workflow to reduce complexity and enhance efficiency.
Program Management
Based on experience and simple math, we were capable of estimating part cost at the onset of the project allowing for proper budgetary planning.
Developed a detailed summary of the project, including milestones, product cost targets, pre-engineering tooling costs, and estimated testing costs.
Engaged with partner vendors early to ensure realistic, real-world cost projections.
Project Management
Managed the project timeline from start to finish, assigning roles and responsibilities of both internal and external resources.
Regulatory Compliance
Operated within the company’s Quality Management System (QMS).
Supported and provided regulatory documentation and work instruction development.
Industrial Design
Sketched initial concepts and built CAD models.
Down-selected designs based on human factors, environmental needs, and product workflow.
Developed graphics, color material finish and branding.
Final designs balanced form, fit, and function.
Engineering
The HaA engineering team designed multiple iterations of the device and worked closely with high capability contract manufacturers to make sure the device could be made repeatedly and within specifications.
CAD Development
Developed detailed 3D CAD models based on final product specifications.
Prototyping
As part of our prototyping services, we built fully functional units for testing, and to fuel stakeholder confidence.
HaA prototyped parts using various methods including 3-D printing, laser cutting, and CNC machining. The team worked closely with the final component suppliers to make sure prototype designs were easily transferable to manufacturing.
Testing
HaA worked closely with accredited calibration and test facilities around the country to ensure accurate calibration and requirements-based testing.
Manufacturing Support
Identified appropriate extrusion, catheter assembly, and injection molding manufacturers for scalable production.